Prof. Zheng Weitao's team from Jilin University team, JACS: Spatially Confined PdHx Metallenes for Efficient Hydrogen Evolution
Recently, the team of Professor Zheng Weitao and Professor Cui Xiaoqiang from the School of Materials Science and Engineering of Jilin University has made new progress in the field of metastable and low-dimensional metal materials. The research result paper titled "Spatially Confined PdHx Metallenes by Tensile Strained Atomic Ru Layers for Efficient Hydrogen Evolution" was published online in the Journal of the American Chemical Society on March 5, 2023.
Metallene is a class of metastable two-dimensional metal materials with atomic-level thickness and graphene-like structure, which has attracted extensive attention from researchers in the field of energy catalysis due to its ultra-high surface area and the resulting large number of unsaturated metal atoms. Alloying light atoms (especially hydrogen) with metallene can change the arrangement of metal atoms and optimize its electronic structure and enhance intrinsic activity. However, the unsaturated coordinated metal atoms in the metallenes attract each other and produce squeezing strain, which results in that the gap hydrogen atoms easily escape from the crystal lattice, destroying their intrinsic structure and restricting subsequent applications.
To solve this problem, our work proposes a new strategy to stabilize interstitial hydrogen atoms through the spatial confinement effect of the Ru atomic layer, significantly improving the stability of PdHx metallenes: PdHx@Ru metallenes can withstand temperatures of more than 200 °C under ambient conditions, while PdHx-metallenes exist stably below 60 °C. More importantly, the prepared PdHx@Ru metallenes have excellent HER activity at a current density of 10 mA cm−2 in alkaline electrolyte, whose overpotential is only 30 mV, which is better than most reported Ru groups hydrogen evolution catalyst. At the same time, it also has a good stability and negligible activity decay after 10,000 cycles. Combining control experiments and theoretical calculations reveals that PdHx@Ru is a highly active source of metallenes: kernel PdHx Tensile strain is induced in the surface Ru atomic layer, which enhances the adsorption of H2O and reduces the energy barrier of H2O dissociation, as well as provides moderate hydrogen adsorption energy for accelerating the generation and desorption of hydrogen.
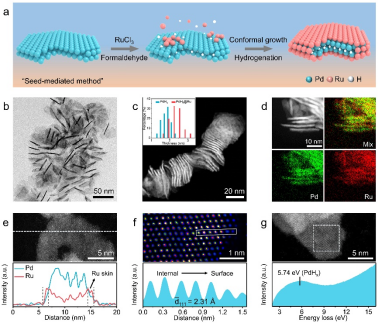
Figure 1: Morphological characterization of PdHx@Ru metallenes

Figure 2: Stability study of PdHx@Ru metallenes
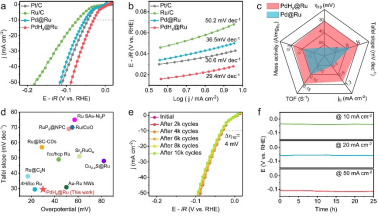
Figure 3: Hydrogen evolution properties of PdHx @Ru metallenes
“DingXin scholar” postdoctoral Fan Jinchang and postgraduate student Feng Zhipeng, majoring in materials physics and chemistry, are the co-first authors of this paper. The corresponding authors are Professor Zheng Weitao and Professor Cui Xiaoqiang from the School of Materials Science and Engineering, Jilin University. This work was supported by the National Natural Science Foundation of China (NSFC) General and Key Programs.
Link to the full text of the paper: https://pubs.acs.org/doi/10.1021/jacs.2c11692